New therapeutics could overcome blood-brain barrier
Developing therapeutics can be challenging due to a blood-brain barrier while delivering drugs.
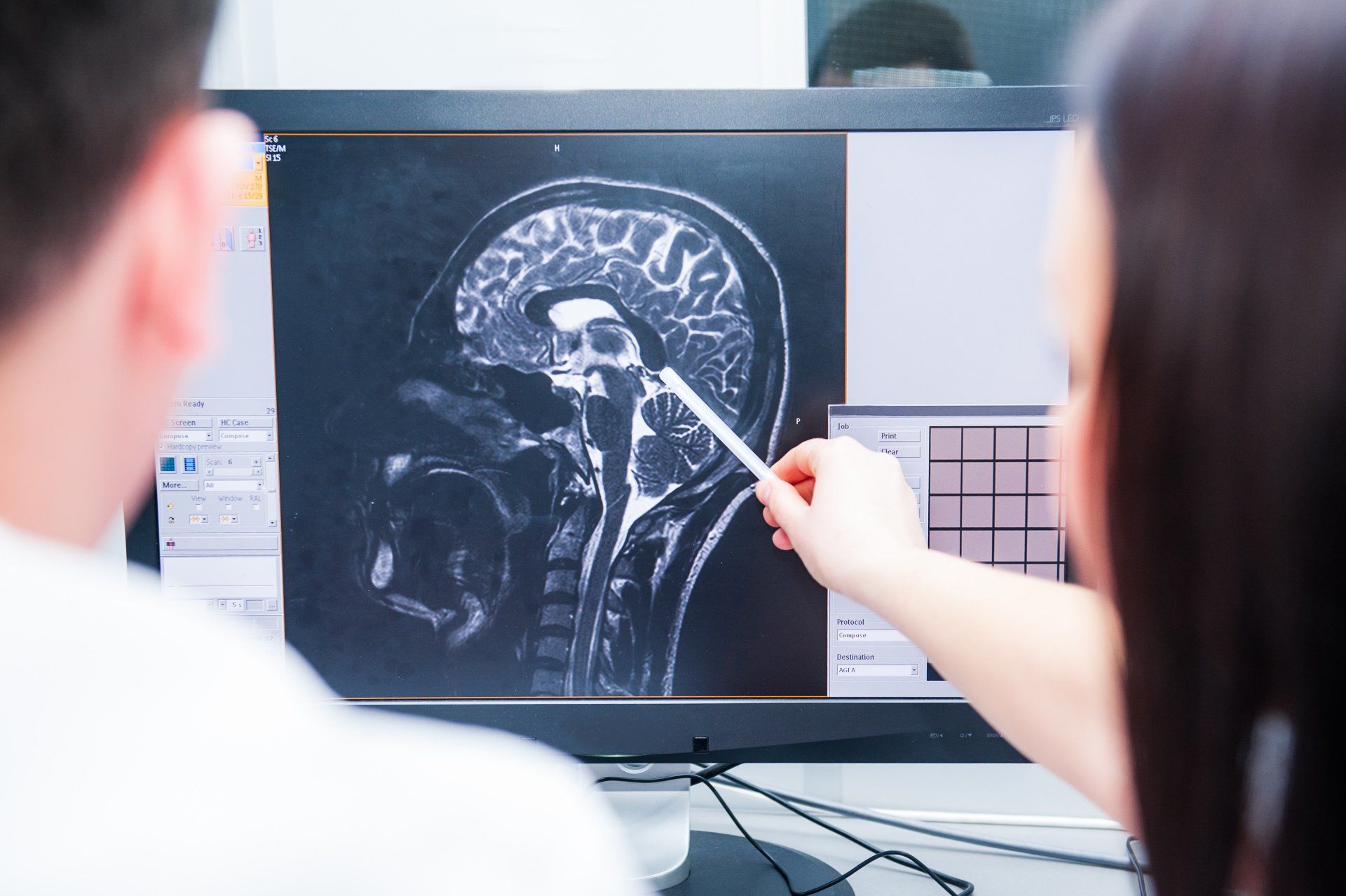
Traumatic Brain Injury or TBI is sustained from severe or mild damage to the brain tissue from falls, vehicle crashes, sports injuries, various accidents or assaults.
In North America, more than 1.7 million people suffer annually from TBI and the consequent medical care costs exceed USD 70 billion, according to a press release by Persistence Market Research posted on the Med Gadget website.
Persistence Market Research (PMR) is a third-platform research firm. The company’s research model is a unique collaboration of data analytics and market research methodology to help businesses achieve optimal performance.
According to The World Health Organization (WHO), about 10 million people suffer a TBI each year.
Unlike some organs, brain tissue can’t repair itself easily, making it highly vulnerable to injuries and often leading to permanent loss or disability of motor functions. Many who suffer a TBI experience a dramatically reduced quality of life.
“Most neuronal damage occurs sometime after the injury takes place giving a chance for a therapeutic intervention,” the press release reads. “In May 2013, DARPA awarded USD 6 million to develop nanotech therapy for combating TBI to a team from University of California San Diego. Nanotechnology offers chances to overcome this physiological challenge of blood-brain barrier to deliver therapeutics.”
These silicon nanoparticles are porous, so they can carry a significant drug load through the blood brain barrier in order to treat TBI patients.
“Cognosci, a privately held start-up from North Carolina, U.S. has novel therapeutic compounds in pipeline for the treatment of TBI, which the company expects to launch in coming years with a strategic partnership alliance,” the release reads. “Currently there are two notable clinical trials for gauging the capability of progesterone for traumatic brain injury. The ProTECT III (progesterone for traumatic brain injury ) is currently conducted by the Emory University and is sponsored by the NIH. … SyNAPSe, initiated by BHR, a branch of Besins Pharma … is another Phase III study investigating the efficacy and safety of progesterone in patients with severe traumatic brain injuries.”
Currently there are no approved therapeutic products, so the sector is teeming with opportunities. Increasing incidences of TBI due to rising assaults, accidents and military combats are expected to drive the growth of the market.









Request Your Free eBook
Our office has provided information regarding the different types of Wisconsin Personal Injury Accidents that we have experience handling.
Wisconsin Accidents
Wisconsin Personal Injuries
Ready to get started?
Call us at 414-374-4444
Main Office Location
Rozek Law Offices, SC
3970 N Oakland Ave Ste 604
Milwaukee, Wisconsin 53211
Additional Client Meeting Location
Rozek Law Offices - Madison
2810 Crossroads Dr Ste 4046
Madison, Wisconsin 53718
Recent Blog Posts










